 |
| Mille crepe. By Laitr Keiows - Own work, CC BY-SA 3.0, |
The soil we stand on is like a mille crepe, a layered cake made by the slow deposition of solid matter on an ocean bottom, each era adding a layer of a different nature. The process that makes particles even slightly denser than water settle down is called sedimentation.
A particle is pulled down by gravity, slowed down by the viscosity of the solvent. It also gets kicked randomly by the atoms around. For a large and heavy particle like a canon ball this random motion is negligible and the particle sediment to the bottom. For a small and almost buoyant particle like a protein, this random motion dominates and the particle diffuses in any direction. In between we have the so called sedimentation-diffusion equilibrium. Particles settle down, but also diffuse up, and we observe that the concentration of particles changes depending on height. At the bottom we count more particles than at the top. This is what we call a density profile.
Equilibrium density profiles are a great tool for physicist. By measuring them, you can learn how your particles behave as a system. For example, if you observe a density that decreases exponentially with altitude, you known that the suspension behaves like an "ideal gas", which means that the particles almost do not interact. That's more or less the density profile of the gases in the atmosphere.
If you observe a sudden jump in a density profile, it means that you have an interface between two phases. For example between a gas of particles and a liquid of particles.
| A colloidal gas-liquid interface. Picture by Paddy Royall. |
There are several ways to get to this triple coexistence situation. One possibility is that you first have the gas and the liquid that separate, and then the crystal forms from the liquid. A second possibility is crystals condensing from the liquid, settling down in sufficient quantities and only then does the liquid evaporates to form a gas layer on top. A third possibility is the crystals forming at the same time as gas bubbles, racing to the bottom or the top respectively. Only when gas and crystal layers sit on top of each other does some of the crystal melts to form a liquid layer in between.
My contribution was to add some more complexity to the first scenario. What if I add a few large particles (green) in the suspension of small particles (red) ?
At first, nothing changes: on top a gas that has almost no particle and on the bottom a mixture of many small and a few large particles. If there was only small particles crystals would form at the bottom. But the large particles get in the way and no crystallisation occurs at the bottom.
Meanwhile the large particles settle faster than the small ones. So at the top of the liquid we soon have a layer devoid of large particles. Only small particles? Easy to make crystals then (big red blobs on the video below). Crystals are large, compact, and fall even faster than large particles. They outpace them and dive in the dense mixture of large and small particles. Splash!
And here we have something unexpected: the crystals float! I mean, yes, ice floats over water, we are accustomed to this. But water is an exception. Solid metal sink down into molten metal.
Actually we demonstrates that the mixture of small and large particles can get so dense without crystallizing that crystals made only of small particles can float in it.
The crystals are reasonably happy in there, not melting but not growing either. Since crystals are dropped continuously from the top, they end up filling pretty much the whole pool (where the large particles are) and even piling up over the level of the large particles.
Now the crystals that are over the level of the pool have no large particles to prevent their growth, so they grow and make a dense "ice pack" on top of the pool.
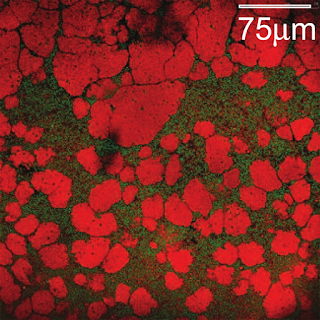 |
| Final state of the limit between floating crystals (below) and the ice pack (above). This is the same place as the video above. |
At the end, you get a pretty layered cake: gas on top, then a layer of liquid, then the ice pack, then the crystals made of small particles floating in the pool of large and small particles.
 |
| Details of the full layered sediment. Top: gas-liquid interface. Middle: ice pack. Bottom: crystals made of small particles in a small+large amorphous matrix. |
Leocmach, M., Royall, C. P., & Tanaka, H. (2010). Novel zone formation due to interplay between sedimentation and phase ordering. EPL (Europhysics Letters), 89(3), 38006. doi:10.1209/0295-5075/89/38006
http://arxiv.org/abs/1402.0315


No comments:
Post a Comment